In this article I address four (4) different aspects for you to consider, when aiming to improve your ways of working. The context is medical device product development (incl. software) and the regulatory requirements of the industry – meaning the needed design control, safety risk management (ISO 14971) and the compliance to standards, FDA and other regional bodies.
At the end there is a link to our case study, in which a leading medical device company tells about the benefits they have achieved on their path.
1. Identify the core working processes and their interactions
Find the most relevant working processes that are in the center of your medical device and software development. The important thing is to see how those processes relate to each other. How people in different functions and their information need to interact? Some interactions are tighter than the others. What pieces of information relate to each other naturally?
Your existing traceability matrices are a good source for finding interactions. What process areas are involved with the information you need to trace? In the big picture your working process landscape might look something like this:
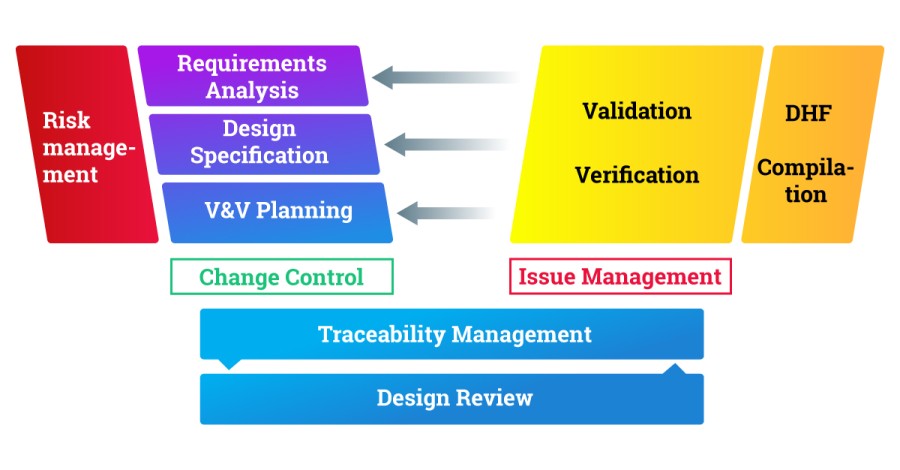
In your company the exact processes or their names may differ, and you might find other areas relevant for you.
The following points may help you identify the process relations. What is needed:
– for different roles to collaborate (requirements analyst, safety expert, test engineer, etc.)
– to derive their work based on the work of the other roles
– to connect their information, e.g. as the traceability matrix demands
2. Find the right granularity of information
Traditionally, documents and files have been the basic units of information – and they still are, at least for regulatory bodies. But when you look at your traceability matrix: are the individual references documents? – No, but they refer to document sections, Excel rows, database objects or file references. The pieces of traced information go inside documents or files.
Another finding is that some of your documents may be artificially small. If you have a document for one “engineering change” or “CAPA”, it is not a real document but a placeholder to get a document identification and a link for it.
What are your basic information elements in each of the process areas above? This gives you an information model, which helps in seeing if your current implementation is really based on the real basic information elements or something else.
3. How can you build quality in?
Build quality in – this is a well-known lean development rule, and it strongly applies here. Quality manual is the usual corner stone. However, usually it’s sitting in a cabinet, which means it’s not really tied into the actual doing. You could think whether it is possible to enforce the medical quality practises and SOPs as part of the normal R&D work, driven with clear workflows.
If you think of the process areas of the aspect 1 and the information elements discovered with the aspect 2:
– Can you facilitate people to carry out their work relying on defined workflows?
– Do they need to remember a lot of things, or do the workflows guide them forward?
– Can you cut down manual work related to the workflows and quality checking?
4. How can you digitalize?
It is quite obvious that reviewing and approving documents with electronic signatures is much faster than signing papers. The next step is to consider, whether there are (semi)manual steps in the e-signing. Do you need to produce information from multiple systems to get it signed somewhere else? What is your coverage with e-signatures when considering the process areas (aspect 1), info elements (2) and workflows (3)? How much rework is needed, if something is not approved?
Digitalization of R&D processes does not only mean e-signatures. It means facilitating working processes so that people can collaborate real-time on the latest master data. Digitalization makes automation possible, which saves time and reduces errors. It helps people concentrate on the essential, instead of doing something manually – such as copy-pasting progress graphs to a PowerPoint report and sending it by email.
For finding the potential for digitalization you can assess anything that currently takes manual work. Here are some candidates:
– Reviews and making records of those
– Gathering information for signing
– Updating traceability matrices
– Change control and taking care of change impacts
– Compiling DHF (Design History File)
– Reporting verification and validation results
– Reporting status and progress
– Relaying information for any collaboration purpose
– Reusing information (e.g. standard requirements to be used in a new project)
As a Summary:
1. What are the process areas to consider first?
2. What is the real information model like?
3. How can you define better ways of working, taking the advantage of workflows?
4. How can you support all the previous with modern digital tools?
Read a case study what Planmeca has achieved by developing their ways of working.
Author: Pasi Ahola, Managing Director, Taipuva Consulting Oy
For an example of the capabilities of a modern digital tool, see the video below (1 min 37 s):
To learn even more…
Watch a webinar recording below. It’s about facilitating medical device design control with the help of Polarion ALM.
This is the first part in a three-part webinar series that introduces “Intelligent Design Control” of mechatronic medical devices. You will see key features that streamline and automate the process, and maintain critical traceability when building an electronic Design History File (eDHF).